Regarding the Covid "vaccines"
A purely scientific perspective
After a brief discussion with a healthcare worker in a doctor’s office in early 2021 regarding the COVID shots, I decided I needed to put together a brief article to help people understand why the shots that were being manufactured could not possibly prevent infection with the SARS-CoV2 virus, and why I thought they were (and have now proven to be) actually dangerous. Well, as often happens, I set about my research looking to the manufacturers’ own studies and other studies published on the National Institutes of Health website and other reputable sites, and my brief article was no longer brief. Then, before sharing it publicly, I wanted it reviewed by doctors. So, I sent it to a few doctors I know, as well as some I don’t but who were vocal about their opposition to pushing these injections out so quickly. Many doctors never responded, though I am very thankful to Dr. Mike Yeadon for at least giving it a cursory reading and for his kind words about it, and it took a while for others to respond, so my paper was not shared until a couple of months after it had been written and others were starting to notice the same issues to which I had alluded. Due to that time gap, shortly before “publishing”, I also updated numbers gathered from VAERS to reflect what at the time were the current numbers.
Since it was published on another website under a slightly modified title, I thought I’d share it here under its original title with only slight modifications to the format. This is likely too long for email, so you may need to read this on the substack website. All of the references are still intact as end notes (and there are a lot of them).
I know it’s pretty late in the game at this point, but I think it’s worthwhile to post this here as it may help provide questions to ask yourself when a new pharmaceutical is proffered as some type of miracle drug or as the answer to a major problem. So, without further ado…
A Brief Overview of the COVID-19 “Vaccines”
There is a major push to get as many people as possible to take the COVID-19/SARS-CoV-2 (SARS-CoV-2 is the actual virus that causes the COVID-19 “disease”) shots, from government, from pharmaceutical companies, even from pharmacies. The urgency with which this is being approached may be likened to propaganda. Marketing, peer pressure, the threat of being blocked from services, all of these are tools being used to manipulate people into rolling up their sleeves and taking a needle. In order to understand this, several questions really need to be answered. What is the COVID-19 “vaccine”? What tests have been performed to evaluate the efficacy and safety of these shots? Is it truly safe and effective? Why is there such a strong push to get as many people as possible to take the shot?
You may wonder, why I am putting “vaccine” in quotes with regard to the COVID-19 shots. To answer this, referring to the traditional historical definition of a vaccine is necessary.
The quick reference definition of “vaccine” from the Oxford Dictionary of Biochemistry and Molecular Biology is:
Any preparation of immunogenic material suitable for the stimulation of active immunity in animals without inducing disease. Vaccines may be based on dead or attenuated microorganisms; altered toxins (toxoids); or viruses[1]
Likewise, from the Oxford English Dictionary:
A substance used to stimulate the production of antibodies and provide immunity against one or several diseases, prepared from the causative agent of a disease, its products, or a synthetic substitute, treated to act as an antigen without inducing the disease.[2]
According to the CDC:
Vaccines: The Basics
Vaccines contain the same germs that cause disease. (For example, measles vaccine contains measles virus, and Hib vaccine contains Hib bacteria.) But they have been either killed or weakened to the point that they don’t make you sick. Some vaccines contain only a part of the disease germ.
A vaccine stimulates your immune system to produce antibodies, exactly like it would if you were exposed to the disease. After getting vaccinated, you develop immunity to that disease, without having to get the disease first.
This is what makes vaccines such powerful medicine. Unlike most medicines, which treat or cure diseases, vaccines prevent them.[3]
There are three common points in all of these definitions:
A vaccine is prepared from an attenuated (weakened) or killed pathogen itself, a portion thereof, or a product (for instance, a toxin) the pathogen produces, or a synthetic version of any of these in such a form that it is unable to replicate or cause disease.
A vaccine acts as an antigen to stimulate an immune response. An antigen is a substance that directly stimulates an immune response such as the production of antibodies (antibodies attack the antigen).
A vaccine creates immunity to a specific disease, it prevents the vaccinated person from being contracting the disease and it prevents the vaccinated person from transmitting the disease to others.
While some organizations are now modifying their definition of “vaccine” to include other substances and outcomes (such as minimizing symptoms rather than actually stimulating true immunity), this is the historical definition of vaccine. This also is why the flu “shot” is generally called a “shot” and not a “vaccine” – it does not confer immunity. How does the COVID-19 shot fit within this definition? Let’s approach each point individually.
Is the COVID-19 shot prepared from an attenuated or killed virus, or a portion of it, or even a product of it that is unable to replicate? No. The COVID-19 shots are produced in two different manners. The Moderna[4] and Pfizer[5] shots contain mRNA encoded for a SARS-CoV-2 spike protein wrapped in a nano-lipid particles (extremely tiny fat particles). These are called mRNA “vaccines.” The Janssen/J&J[6] and AstraZeneca[7] shots contain a laboratory-modified version of another human virus (adenovirus – the same type of virus that causes the common cold) encoded for the SARS-CoV-2 spike protein. These are called viral vector “vaccines.” While some may argue this mRNA or coding in the viral vector delivery system is a synthesized portion of the SARS-CoV-2 virus, these vaccines are formulated in such a way that they need to enter the body’s cells and use the body’s cells to replicate the protein encoded in the mRNA. In other words, rather than exposing the body to an antigen, they use the body to generate an antigen (this is exactly how viruses themselves work – by hijacking the cell’s protein replication mechanism in order to replicate itself and/or to produce toxins).
Does the COVID-19 shot act as an antigen to stimulate an immune response? No. A traditional vaccine exposes the patient to the actual pathogen or toxin, which in the body is expected to directly stimulate the immune system to develop specific defenses against that pathogen or toxin. The COVID-19 shots instead use their delivery systems (the nano-lipid particles or modified adenovirus) to enter cells and, using the mRNA, cause the cells to produce the toxic spike protein (the antigen) in hopes that the body will then react to the antigen by developing antibodies. This process will be discussed further in addressing the efficacy of the COVID-19 shots.
Does the COVID-19 shot confer immunity to the recipient and prevent the recipient from transmitting COVID-19 to others. No. Though the manufacturers make impressive claims from the results of their clinical trials, even the CDC uses less powerful language with regards to their effectiveness:
What We Know about How Well COVID-19 Vaccines Are Working
COVID-19 vaccination reduces the risk of COVID-19 and its potentially severe complications. All COVID-19 vaccines currently authorized for use in the United States helped protect people against COVID-19, including severe illness, in clinical trial settings. So far, studies that have looked at how COVID-19 vaccines work in real-world conditions (vaccine effectiveness studies) have shown that these vaccines are working well.[8]
Notice they state that the shot “reduces the risk of COVID-19”, not that it provides immunity to COVID-19. There is a big difference. Even the manufacturers themselves often use vague language, often relying on words such as “can” and “may” with regard to preventing illness. For instance, AstraZeneca’s vaccine information document states the following:
COVID-19 Vaccine AstraZeneca stimulates the body’s natural defences (immune system). It causes the body to produce its own protection (antibodies) against the virus. This will help to protect you against COVID-19 in the future.[9]
Janssen’s website states the following:
The Janssen COVID‑19 Vaccine is an unapproved vaccine that may prevent COVID‑19.[10]
Limitations of Vaccine Effectiveness: The Janssen COVID-19 Vaccine may not protect all vaccinated individuals.[11]
Moderna’s published information regarding their mRNA vaccine states:
The Moderna COVID‑19 Vaccine has not yet undergone the same type of review as an FDA‑approved or cleared product. FDA may issue an "emergency use authorization" when certain criteria are met, which includes that there are no adequate, approved, and available alternatives. In addition, the FDA decision is based on the totality of the scientific evidence available showing that the product may be effective to prevent COVID‑19 during the COVID‑19 pandemic and that the known and potential benefits of the product outweigh the known and potential risks of the product.[12]
Despite how proud all of the manufacturers are of their products in declaring efficacy numbers, some claiming efficacy upward of 90%, none of these companies make very solid statements regarding effectiveness. The manufacturer claims and documents also, as in the Moderna statement above, make it very clear, as they are required to by regulation, that these shots are not approved by any regulatory agencies, but instead are being administered under Emergency Use Authorization.
Emergency Use Authorization vs. Approval
Many people don’t realize that none of the COVID-19 shots are approved by any regulatory agencies, even outside the United States. Often the media and even government spokespeople further this confusion by mistakenly using the word “approved” when speaking of the shots. What’s the difference? Well, what do the manufacturer sites say?
The Janssen site has a couple of notices, depending on the page. One of the longer of their notices states the following:
The Janssen COVID-19 Vaccine has not been approved or licensed by the U.S. Food and Drug Administration (FDA), but has been authorized by FDA through an Emergency Use Authorization (EUA) for active immunization to prevent Coronavirus Disease 2019 (COVID-19) in individuals 18 years of age and older. The emergency use of this product is authorized only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of the medical product under Section 564(b)(1) of the FD&C Act, unless the declaration is terminated or authorization revoked sooner.[13]
The notice on one of AstraZeneca’s pages reads:
COVID-19 Vaccine AstraZeneca has been given ‘emergency use listing’ by the World Health Organization. This means that there is more evidence to come about this medicine. The World Health Organization will review new information on this medicine as it becomes available and the leaflet will be updated as necessary.[14]
It is important to note their recognition that “there is more evidence to come.” That statement does not preclude such evidence from providing information that counters the current efficacy and safety narrative.
Moderna’s pages sport the following disclaimer:
The Moderna COVID‑19 Vaccine has not been approved or licensed by the US Food and Drug Administration (FDA), but has been authorized for emergency use by FDA, under an Emergency Use Authorization (EUA), to prevent Coronavirus Disease 2019 (COVID‑19) for use in individuals 18 years of age and older. There is no FDA-approved vaccine to prevent COVID‑19.
The EUA for the Moderna COVID‑19 Vaccine is in effect for the duration of the COVID‑19 EUA declaration justifying emergency use of the product, unless the declaration is terminated or the authorization is revoked sooner.[15]
Pfizer also has the standard disclaimer:
The Pfizer-BioNTech COVID-19 vaccine has not been approved or licensed by the U.S. Food and Drug Administration (FDA), but has been authorized for emergency use by FDA under an Emergency Use Authorization (EUA) to prevent Coronavirus Disease 2019 (COVID-19) for use in individuals 12 years of age and older. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of the medical product under Section 564(b)(1) of the FD&C Act unless the declaration is terminated or authorization revoked sooner. Please see EUA Fact Sheet here.[16]
Pfizer, on their page regarding mRNA “vaccine” technology also has the following statements:
Regulatory authorizations have allowed mRNA vaccines to become one of the key tools in the fight against COVID-19. Millions of people in countries around the world have already received mRNA vaccines against COVID-19.[viii]
These vaccines have been studied in tens of thousands of clinical trial participants, and manufacturers and regulatory bodies will continue to monitor performance, safety, and efficacy of vaccines through a country’s regulatory approval and beyond.[17]
What does all this mean? Why it is important? Under normal circumstances, vaccines go through a fairly rigorous process before receiving approval for commercial use. The FDA’s site lists six general stages for developing a new vaccine[18]:
Exploratory stage
Pre-clinical stage
Clinical development
Regulatory review and approval
Manufacturing
Quality control
The FDA also lists six typical steps for the new vaccine approval process[19]:
An Investigational New Drug application
Pre-licensure vaccine clinical trials
A Biologics License Application (BLA)
Inspection of the manufacturing facility
Presentation of findings to FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC)
Usability testing of product labeling
Managing to get through this process is no small feat, and it may take manufacturers years to complete the process and receive approval. None of the so-called “vaccines” currently in use have been through this process. As a matter of fact, all of the shots are still in phase III clinical trials. These trials being incomplete prior to the shots being authorized for emergency use, and now being widely distributed, has resulted in many phase III trials essentially being invalidated, as the randomization and masking has been compromised. Peter Doshi, senior editor of the British Medical Journal, wrote in May of 2021:
As hundreds of millions of people around the world get vaccinated, it may seem like wordsmithing to highlight the fact that none of the covid-19 vaccines in use are actually “approved.” Through an emergency access mechanism known as Emergency Use Authorization (EUA), the products being rolled out still technically remain “investigational.”[20]
That’s right, all of the COVID-19 shots are still experimental, or in Doshi’s words “investigational.”
Doshi’s article continues:
One key difference between EUA and approval (also called “licensure,” and which for vaccines is known as a BLA (Biologics License Application)) was the expected length of follow-up of trial participants. Unlike its clear articulation of two months for an EUA, the FDA has not committed to a clear minimum for approval.
…
The FDA’s Doran Fink responded: “I couldn’t predict, but I will say that we typically ask for at least six months of follow-up in a substantial number of clinical trial participants to constitute a safety database that would support licensure.”
An approval based on six months of data would represent one of the fastest for a novel vaccine in FDA history. Among the six “first in disease” vaccines approved by the FDA since 2006, pre-licensure pivotal trials were a median of 23 months in duration, according to a recent analysis.[21]
Continued trial participant follow-up that normally lasts in the neighborhood of two years before approval is granted was circumvented to allow emergency use after only two months of participant follow-up. A representative for the FDA stated that they would expect at least 6 months of follow-up (though not necessarily more), only one quarter of the typical/median amount of follow-up, for approval. According to Doshi, even a World Health Organization working group called for at least 12 months of follow-up. Doshi then cites the FDAs formal guidance from June 2020 wanting participants followed “ideally at least one to two years.” Again, some may ask, what does this matter? Trials have shown the shots to be safe and effective, right? After all, the CDC’s website has those words plastered all over its pages, as do manufacturers’ websites. There is a major problem though. As Doshi goes on:
“Very often, it’s the fact that we have that placebo controlled follow-up over time, that gives us the ability to say that the vaccine didn’t cause something at a longer period of time after vaccination,” the FDA’s Philip Krause explained last December.
Yet there is a gap – currently of unknown size but growing – between any expectation of blinded placebo controlled data, and the reality that within weeks of the vaccines receiving an EUA the unblinding of trials commenced as placebo recipients were offered the chance to get vaccinated.[22]
According to Doshi, the BMJ contacted Moderna, Pfizer, and Janssen to find out whether their trials were still blinded and placebo-controlled. Pfizer “declined to say,” Moderna has stated that all placebo participants were offered the vaccine and 98% of those have received it, and Janssen doesn’t have specific numbers regarding how many of their study participants had received the vaccine. Basically, as Doshi states with regard to Moderna, “the trial is unblinded, and the placebo group no longer exists.” This, despite that in October 2020, the FDA said:
Continuation of placebo controlled follow-up after EUA will be important and may actually be critical to ensure that additional safety and effectiveness data are accrued to support submission of a licensure application as soon as possible following an EUA. … Once a decision is made to unblind an ongoing placebo controlled trial, that decision cannot be walked back. And that controlled follow-up is lost forever.[23]
At best, the current widespread administration of these shots should be considered the largest human clinical trial of any medicine or medical treatment in history.
“But if it is safe and effective, what is the harm?” Doesn’t this benefit us all? This is where we need to look at the studies themselves.
Clinical Trial Results and Efficacy Numbers
As I’ve stated, the clinical trials are still ongoing. Manufacturers have, however, already provided many study results. It is from these results that the manufacturers lay claim to efficacy and safety numbers for their shots. What do these studies actually tell us?
Pfizer’s study will be used as an example, as the study details filed with the U.S. National Library of Medicine is fairly rigorous and others follow a similar pattern, though perhaps not as rigorous.[24],[25],[26],[27]
Pfizer’s study began April 29, 2020, and according to their own filing, is expected to be complete on May 2, 2023.[28] Though the completion date is still over a year and a half in the future, multiple articles have been written documenting some of the to-date test results. The conclusion in a study published by the New England Journal of Medicine states:
A two-dose regimen of BNT162b2 conferred 95% protection against Covid-19 in persons 16 years of age or older. Safety over a median of 2 months was similar to that of other viral vaccines.[29]
From where do they draw this conclusion? How do the authors arrive at a figure of 95% for the shot’s rate of protection? The laboratories set out to test their mRNA solution by soliciting the participation of over 40,000 people, each of whom would receive two injections 21 days apart, and each of whom would be randomly placed into a group such that half of the candidates would receive the Pfizer shot, and the other half would receive a placebo. Neither the candidates nor the “[s]ite staff who were responsible for safety evaluation” would be aware of which candidates belonged to which group. The safety study relied upon solicited feedback regarding local or systemic adverse events from the participants within 7 days after each dose, feedback which would be prompted by and recorded in an electronic diary. The study also relied upon unsolicited feedback through one month after the second dose for adverse events, and through six months after the second dose for serious adverse events. While this is all well and good, there is a problem with this sort of study: studies that rely on participant feedback, especially unsolicited feedback, tend to be highly misleading. People don’t always provide feedback, even when it’s expected. In the case of something like this, the participants may also, rather than provide feedback, either see a doctor or report an adverse event via another avenue (such as the government’s VAERS database – Vaccine Adverse Event Reporting System). If a participant passed away, but there was no formal follow-up, feedback wouldn’t be received. There are too many variables involved when depending upon participant feedback to consider such statistical information reliable. As a matter of fact, the article itself states, “The findings are descriptive in nature and not based on formal statistical hypothesis testing.”[30] This is a very well-known issue, and not isolated to medical studies, so safety claims should be treated as dubious at best.
Back to the efficacy numbers, according to the article, the primary method for assessing the effectiveness of the shot in preventing COVID-19 was to evaluate participants who showed no evidence of prior COVID-19 infection to see how many developed COVID-19 infection after receiving the second injection, and compare between the placebo and medicated groups:
Among 36,523 participants who had no evidence of existing or prior SARS-CoV-2 infection, 8 cases of Covid-19 with onset at least 7 days after the second dose were observed among vaccine recipients and 162 among placebo recipients. This case split corresponds to 95.0% vaccine efficacy (95% confidence interval [CI], 90.3 to 97.6; Table 2)[31]
University of Minnesota, reporting on the AstraZeneca shot, uses the same calculation:
Of the 11,636 adults, 131 (1.1%) had symptomatic COVID-19 more than 14 days after the second vaccine dose, including 30 of 5,807 (0.5%) in the COVID-19 vaccine group and 101 of 5,829 (1.7%) in the control group, indicating a vaccine efficacy of 70%.[32]
This is all well and good, but there is a serious problem with this construct: in order for this statistic to be meaningful as stated, every one of those participants would need to receive an infective level of exposure to the virus. Though this calculation appears to be common in assessing the efficacy of vaccines based on what is called the “attack ratio” (calculated as: E = 1 - nvi/npi, where E is efficacy, nvi is number of participants receiving the vaccine who become infected after vaccination, and npi is the number of participants receiving a placebo who become infected after receiving the placebo),[33] the method used to arrive at this number is specious (results of clinical studies for the other manufacturers’ shots used the same calculation). If this method were valid, we could use it based on a recent article from BBC News wherein they stated that the number of patients being treated for COVID-19 were growing, and about half of them had chosen not to be vaccinated.[34] This logically means that half of them had been vaccinated. Since the article does not provide a specific figure, let’s assume there are 400 hospitalized (based on the article’s statement that infection rates in the community are at about 400/100,000), Using the above formula, that gives us 100% - 200/200 (half were vaccinated, half were not), which results in 100% - 100% = 0%. So, the vaccine has 0% effectiveness (at least for preventing COVID-19 hospitalization, if not infection itself). While this may seem silly and random, considering those hospitalized provide a reasonably randomized sample of the general population, it is no more an invalid analysis than it is within the clinical trial. In actuality however, both calculations are invalid. The point is, without intentional infective exposure, there are too many variables to produce any reliable statistics in this manner. If the participants were not exposed, then from what would the shot be protecting them? Who is to say the 8 cases among those who received the actual Pfizer shot weren’t the only ones who were exposed? Do these results simply mean that more people in the placebo group were exposed? There is no way of knowing to how many other people each of the participant was exposed and whether any such people were infected or even contagious. Perhaps by chance more of the group receiving the Pfizer shot chose to isolate themselves out of concern for safety. According to the article, PCR tests were used to confirm cases. How accurate are the PCR tests? In reality, this efficacy number means absolutely nothing – it is 100% useless without confirmed, equivalent infective exposure to the virus. A study can neither show nor prove that a person receiving the shot was protected from a virus to which they have not been exposed; to claim otherwise is disingenuous.
Now, if efficacy were the only statistic by which the manufacturer made their claims, the case would be closed and we could say these shots were useless. There are more in-depth studies, however, and those should be evaluated as well.
Continuing using the Pfizer vaccine as our example, we can look at a study using mice and macaques. In February 2021, Annette B. Vogel and her colleagues published their findings in Nature magazine. In the section titled “BNT162b-elicted immunogenicity in mice” they record the following results:
A single immunization using either of the candidate vaccines induced high titres of RBD- and S1-binding serum IgG in a dose-level-dependent manner (Fig. 2a, Extended Data Fig. 3a–d); these titres increased more steeply for BNT162b2. On day 28 after injection with 5 μg BNT162b1 or BNT162b2, geometric mean endpoint titres of RBD-binding serum IgG were 752,680 or 434,560, respectively.[35]
What this is basically saying is that the shot caused elevated serum levels of a type of antibody called IgG. This sounds promising, but somewhat deceptively so. While this would be helpful if the SARS-CoV-2 virus were primarily found in the blood stream (this is where IgG titers tend to be highest and this is where IgG is generally responsible for antiviral activity[36]), SARS-CoV-2 concentrated in the epithelium (mucosal lining) of the respiratory tract (where it causes breathing problems) will not likely be attacked by this antibody. Why? IgG occurs in very low concentrations in the respiratory mucosa. The type of antibody relevant to this part of the body is called IgA.[37],[38] The study does not in any way purport to assess any IgA titers in regard to the vaccination. Perhaps even more important is that “during primary infection or immunization, most antigens first elicit IgM (early antibody) responses.”[39] With initial infection, IgG is produced after IgM. So, why weren’t IgM titers measured? Interestingly, IgG titers rise quickly with reinfection, as a sort of memory immune response.[40] Is it possible that the rapid rise in IgG titers was a result of prior immunity due to previous infection or cross-reactivity with immunity to other viruses? This is what Doctors for COVID Ethics suggest. In a letter they sent to doctors in Europe, these four doctors state:
A certain amount of IgM was indeed detected alongside IgG and IgA in some studies [1,4]. Importantly, however, IgG rose faster than IgM [4], which confirms that the early IgG response was indeed of the memory type. This memory response indicates pre-existing, cross-reactive immunity due to previous infection with ordinary respiratory human coronavirus strains.[41]
Regardless of whether the rising titers of IgG did signify just a response to the initial vaccine injection and not any immune memory or cross-reactivity, the FDA stated in May of 2021 that, “results from currently authorized SARS-CoV-2 antibody tests should not be used to evaluate a person’s level of immunity or protection from COVID-19 at any time, and especially after the person received a COVID-19 vaccination.”[42] Therefore, according to the FDA, this does not indicate immunity.
There is some evidence now that the SARS-CoV-2 may also be a vascular disease and not (just) a pulmonary disease, as the Receptor Binding Domain of the spike protein binds to ACE2 receptors which are abundant in vascular endothelial cells (the cells in the lining of blood vessels).[43] If that is the case, IgG may have some benefit, but it still will not clear the virus; however, contrary to articles that say the spike proteins produced by the cells processing the vaccine are “safe” or act “differently” than the spike proteins on the virus, they may be problematic. If the spike protein is a full copy of the SARS-CoV-2 spike protein (as the manufacturers state), then the RBD (the part of the protein that binds to receptors on cells and allows the virus to enter a cell) is identical. The issue is, the RBD on the spike protein is the place where an antibody needs to attach to be able to prevent the virus from entering a cell. If these spike proteins are released by the cells producing them and get into the bloodstream before the body is able to produce or release targeted IgG, then the free-floating spike protein, which can circulate in the body for weeks following taking the shot[44], will have opportunity to bind to the ACE2 receptors of endothelial cells. Researchers found that through this ability to bind to ACE2, even without viral RNA or the ability to replicate, the SARS-CoV-2 spike protein damages vascular endothelial cells.[45] This could very well explain the post-vaccination cases of thrombosis/thrombocytopenia, myocarditis, and pericarditis.
Another issue is that antibodies such as IgG and IgA are not the body’s primary defense against a viral infection. Earlier than antibodies are released (possibly days earlier) after the onset of a viral infection, T cells (“T” is short for “thymus”, the gland responsible in early development for generating a type of protective cells known as “lymphocytes”) and NK (Natural Killer) cells are involved.[46] NK cells are non-specific (meaning, they don’t target a specific virus) and may be the first involved in attacking virally-infected cells. T cells recognize specific targets. T cells notice when something is not quite right with the infected cells because these cells are trained to recognize the specific protein patterns expressed on the surface of normal cells. When a virus enters a cell, it often causes the cell to express proteins on its surface that aren’t normally present. This is when a T cell is able to recognize the cell that is infected and either release substances called “cytotoxins” that destroy the infected cell, or to call in other defenses that can help destroy the infected cell. Again, there is an issue here in that, if the cell does not express a viral protein on its surface prior to the virus reproducing within the cell, viral progeny may be released even before the T cell has had a chance to recognize that the cell is infected, and many viruses don’t cause cells to express such proteins.[47] Therefore, even though the study indicates there is an increase in (non antigen-specific) CD4+ and (antigen-specific) CD8+ T cells in response to the vaccine, if the viral protein is not expressed on the surface of an infected cell prior to the virus replicating, these T cells will provide little benefit. To their credit, the authors do recognize that impaired expression of antigen from cells could hinder clearance of the virus, and they claim the CD8+ T cells could help alleviate this:
Limitation and clearance of virus infection is promoted by the interplay between neutralizing antibodies that eliminate infectious particles and CD8+ T cells that target intracellular reservoirs of virus. CD8+ T cells may also reduce the influx of monocytes into infected lung tissue, which can be associated with undesirable IL-6 and TNF production and impaired antigen presentation.[48]
Still, the body producing spike protein which may, or may not, be expressed on the surface of a cell is different from it being encountered directly or from the cell replicating virus and the mechanism whereby viral proteins are (or are not) expressed on the cell membrane. What could perhaps make this more convincing would be if they were able in vivo to show cytotoxic T cell-mediated lysis of the cells that were producing the spike protein.
To cap off the study, the scientists infected with SARS-CoV-2 a dozen vaccinated (two different versions of the vaccine) macaques, and nine unvaccinated macaques. Six vaccinated macaques were infected intranasally and six were infected intratracheally. The nine control macaques “received the same SARS-CoV-2 challenge.” This is a fairly small sample, and the sampling of control and vaccinated differs. That said, the results were as follows:
Viral RNA was detected in bronchoalveolar lavage fluid from seven of nine control macaques on day 3; from four of eight control macaques on day 6 after challenge (with one indeterminant result); and from none of the six control macaques that underwent bronchoalveolar lavage at the end of project (EOP; days 7–23 after challenge) (Fig. 4a). Viral RNA was detected in the bronchoalveolar lavage fluid of two of six BNT162b1-immunized macaques on day 3 after challenge, and from none thereafter. Viral RNA was not detected in bronchoalveolar lavage fluid from the BNT162b2-immunized, SARS-CoV-2 challenged macaques at any of the time points we sampled.
…
In nasal swabs obtained on the day after challenge, viral RNA was detected from control-immunized macaques (4 of 9) and BNT162b2-immunized macaques (5 of 6), but not from BNT162b1-immunized macaques (Fig. 4b). In subsequent nasal swabs, viral RNA was detected from some of the control-immunized macaques at each sampling time point (5 of 9 on day 3, 4 of 9 on day 6 and 2 of 9 on days 7–23), from some BNT162b1-immunized macaques at only one sampling time point (2 of 6 on day 6) and from none of the BNT162b2-immunized macaques at any sampling time point. Similar patterns were seen in oropharyngeal and rectal swabs: viral RNA was more often detected in control-immunized macaques than in BNT162b1- or BNT162b2-immunized macaques, and there was more persistence of viral RNA in rectal swabs than in oropharyngeal swabs…
At the time of challenge, SARS-CoV-2-neutralizing titres ranged from 208 to 1,185 in the BNT162b1-immunized macaques and from 260 to 1,004 in the BNT162b2-immunized macaques. Neutralizing titres were below the limit of detection in the control macaques (Fig. 4c, d). The control macaques responded to challenge with infectious virus with an increase in SARS-CoV-2-neutralizing titres, consistent with an immune response to viral infection. However, there was no trend towards increasing SARS-CoV-2-neutralizing titres in response to viral challenge in the BNT162b1-immunized or BNT162b2-immunized macaques, consistent with their immunization suppressing SARS-CoV-2 infection. The maximum SARS-CoV-2-neutralizing titre elicited by virus challenge of control macaques remained below 150 through to the time of necropsy, whereas all immunized macaques maintained neutralizing titres greater than 150 throughout the challenge experiment.
None of the challenged macaques—whether immunized or not—showed clinical signs of illness (Extended Data Fig. 7c–f).[49]
A lot of information there, but it was worth looking at. Some (not all) of the control group showed presence of virus in both bronchoalveolar lavage and via nasal swab for several days after being infected, but then it cleared. Likewise, some vaccinated macaques showed viral presence even 6 days after being infected. The control group showed a proper, natural immune response, while the measured immune titers were already so high in the vaccinated group that they showed no increase. This being the case, how long do such titers remain increased in someone who has received the shot, and can such an increase potentially lead to other adverse effects such as Antibody Dependent Enhancement (ADE)[50],[51],[52] of a future infection? (the authors of the study posited that their test results did not appear to support development of ADE in response to the shot). Where are the studies showing the length of time over which these titers remain elevated? If there is any positive effect to having such high titers (many times those stimulated by a natural immune response), do the benefits only last so long as those titers remain elevated? Does any potential protection offered diminish as the titers decrease or approach normal levels? Are memory T cells produced that can stimulate an immune response in the event of future infection? This last is impossible to answer, as all of the test animals were necropsied after the test, so they cannot be virus-challenged again in the future to determine any lasting benefits of the shot. That the control macaques were able to clear the virus almost as well and as quickly as the vaccinated macaques makes any effect of the shot appear negligible and makes difficult any objective conclusion regarding potential benefits of the shot. Based on the insignificant differences between the control and vaccine groups, no reliable inferences can be made about vaccine effectiveness in preventing disease, and certainly no confident assertions may be made regarding the vaccine preventing serious illness, as all of the macaques, including the control group, exhibited no clinical symptoms. Which leads to further questions:
1. Was the virus challenge effective? Were the macaques actually infected?
2. If they all were infected, how infectious to healthy subjects is this virus?
3. Is it just in people advanced in age or with underlying conditions that infection is problematic?
4. Did the vaccine provide any benefit over a natural immune response?
5. Are the PCR tests for the presence of virus accurate?
If we answer yes to question number 1, and the study appears to indicate they were, then the answers that follow are clear – the virus isn’t very dangerous to healthy subjects (much like influenza), far less so than the government and health authorities would have us believe, it is likely only a problem for those well advanced in age and/or with serious underlying conditions (much like influenza, which has been fairly evident if one is able to weed through the media hype), and the so-called vaccine does not provide any significant benefit over natural immune response. If anything, the vaccine is likely more dangerous than helpful due to the potential effects of the spike protein to ACE2 receptors and to potential ADE in the vaccinated being infected post vaccination. Even the CDC has had to admit to the cause/effect relationship between the vaccines and serious adverse events such as the previously listed thrombocytopenia, myocarditis, and pericarditis (as well as death).[53]
A discussion of the PCR tests will follow in the next section, after a brief summary regarding the article, because there is still something to address with the study. Unfortunately, not only does the study fail prove the effectiveness of the shot, the study methods leave much to be desired. According to the sections “Methods” and “Statistics and Reproducibility”:
No statistical methods were used to predetermine sample size. The experiments were not randomized, and investigators were not blinded to allocation during experiments and outcome assessment, except for the performance of serological assays of nonhuman primates and RT–PCR-based viral load measurements and the interpretation of radiographs, computed tomography scans, and histopathology specimens.
…
No statistical methods were used to predetermine group and samples sizes (n). All experiments were performed once. P values reported for RT–qPCR analysis were determined by nonparametric analysis (Friedman’s test) based on the ranking of viral RNA shedding data within each day. PROC RANK and PROC GLM from SAS 9.4 were used to calculate the P values. All available post-challenge bronchoalveolar lavage fluid and nasal, oropharyngeal and rectal swab samples from the necropsied macaques and all available post-challenge samples through day 10 from the macaques that were not necropsied were included in the analysis. Indeterminate results were excluded from this analysis.[54]
No statistical methods. No randomization. No blinding. No reproducibility (tests were performed only once). Instead of rerunning tests, indeterminate results were simply excluded. As a final point, there are conflicts of interest present - each of the three authors works either for BioNTech or Pfizer. This study is not only not convincing, it does not conclusively show effectiveness of the Pfizer/BioNTech COVID-19 shot, and it is important to note that the authors have a vested interest in proving their product works.
Studies for the other vaccines, where they can be found, are similar. They do not, and cannot, tell us whether these experimental shots have the ability to save anyone’s life. Peter Doshi agrees. In another article in the British Medical Journal, he writes:
None of the trials currently under way are designed to detect a reduction in any serious outcome such as hospital admissions, use of intensive care, or deaths. Nor are the vaccines being studied to determine whether they can interrupt transmission of the virus.[55]
As a final point, some have attributed the current rise in cases, especially in “breakthrough infections” to the delta variant. While viruses mutate and can pose challenges for immunity, the rate at which they mutate and which part of the virus mutates (such as the Receptor Binding Domain) is what matters most. If the RBD does not mutate, and the virus does not develop a new mechanism by which it suppresses protein expression on the surface of the infected cell, then mutations in the virus should not permit it to evade an immune response any more than the original strain of the virus. Regardless, it seems common to point to the new variant as the reason for breakthrough infections being seen in people who have received the shot. However, a study published in the New England Journal of Medicine claims there is little difference in the effectiveness of the shots between the alpha (original) variant and the delta variant:
Only modest differences in vaccine effectiveness were noted with the delta variant as compared with the alpha variant after the receipt of two vaccine doses. Absolute differences in vaccine effectiveness were more marked after the receipt of the first dose. This finding would support efforts to maximize vaccine uptake with two doses among vulnerable populations.[56]
Herein lies another issue with the shots, and that is, that they are singularly focused. Since the shots are designed solely to generate copies of the SARS-CoV-2 spike protein, the shots can only encourage the body to produce antibodies specific to this portion of the virus. With natural immunity developed through infection, the body can actually learn to recognize and fight other parts of the virus, making the coverage offered by a natural immune response broader and more apt to provide protection against new variants. If the shots are losing efficacy such that recipients need a booster, then it can be reasoned that any benefit conferred by the vaccine is based on quickly elevated titers of antibodies that are also rapidly waning, and that the vaccines are not producing the long-term “memory” immune response needed to fight future infection (though IgG, which is a memory antibody, is the primary antibody that was measured in animal trials). If, however, they are ineffective in combating new variants of the virus because the RBD of the spike protein has mutated, then the boosters being suggested by health authorities are pointless, as all they will do is further elevate titers of antibodies that will not bind to the virus. Either way, the only conclusion we can draw from this is that the breakthrough infections would happen with the original virus as well as with the variant, and the shots are equally ineffective against both.
Testing for Covid
Another major factor in how this virus outbreak has been handled is testing. What is a “case”? How do we know if someone has COVID-19? What is a PCR test and how does it work?
From the outset of the virus outbreak, we heard talk of “confirmed cases.” What is a “case”? Generally, a “case” of a disease (for surveillance purposes) is a person presenting with clinical symptoms of that disease, and a “confirmed” (or “laboratory confirmed”) case is a case presenting with symptoms that is confirmed by laboratory testing. These are definitions given even by the CDC.[57],[58] So, technically, a probable COVID-19 case is someone displaying symptoms (i.e. fever, coughing, shortness of breath, loss of sense of taste or smell, etc.), and a confirmed COVID-19 case is someone displaying symptoms and who has had laboratory tests (i.e. x-rays, bloodwork) confirming that the virus is present or having “a direct epidemiologic link to a laboratory-confirmed case.”[59] What we have heard a lot about are “cases” (or even “confirmed cases”) that are “asymptomatic” (without clinical symptoms). Why? What makes these “cases”? This is where the PCR test comes in. The primary test being used to determine or confirm COVID-19 infection is the Polymerase Chain Reaction (PCR) test. The PCR test amplifies nucleic acids present in a lab sample, most often in the case of COVID-19, a nasopharyngeal swab (a swab of the nasal passage where it turns down toward the throat), since this is where respiratory viruses often accumulate. A somewhat simplified explanation of how a PCR works is as follows:
To amplify a segment of DNA using PCR, the sample is first heated so the DNA denatures, or separates into two pieces of single-stranded DNA. Next, an enzyme called "Taq polymerase" synthesizes - builds - two new strands of DNA, using the original strands as templates. This process results in the duplication of the original DNA, with each of the new molecules containing one old and one new strand of DNA. Then each of these strands can be used to create two new copies, and so on, and so on. The cycle of denaturing and synthesizing new DNA is repeated as many as 30 or 40 times, leading to more than one billion exact copies of the original DNA segment.[60]
One point that this explanation leaves out is that the sample is put into a solution containing “primer” that must contain the building blocks for the DNA/RNA the PCR is intending to amplify and/or detect. In order to be truly specific for a particular virus, the RNA being amplified needs to be unique to that virus; therefore, the primer is extremely important. As well, the more cycles through which a sample is run results in greater amplification, but it can also result in detection of something that isn’t actually there. This is the reason most proper testing protocols will state that if the virus for which the test is being run is not detected in significant amounts after 25 cycles, the virus shouldn’t be considered there. Running too many cycles will create erroneous results. This is precisely what has been happening with the PCR tests used for detecting COVID-19. The CDC’s testing protocol calls for 45 amplification cycles with a cycle threshold of 40[61], a number of cycles sufficiently high to “detect” almost anything. Running PCR tests with this high of a cycle threshold will result in a large number of false positives, perhaps even the majority of tests. This isn’t the only problem with the testing protocols. Most people are unaware, but even the PCR tests being used for detecting and diagnosing COVID-19, like the “vaccines”, are not FDA approved but are being used under Emergency Use Authorization.[62],[63] As a matter of fact, the CDC has requested an end to the EUA as of December 31, 2021.[64] There are further problems as well. Twenty-two doctors got together and wrote a critique of the Corman-Drosten Report[65] that is the foundation for the testing being used. In it, they outlined ten particular issues with the protocol, the first eight of which are of key importance and show the testing protocol to be utterly ineffective for detecting live or infections SARS-CoV-2:
1. There exists no specified reason to use these extremely high concentrations of primers in this protocol. The described concentrations lead to increased nonspecific bindings and PCR product amplifications, making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus.
2. Six unspecified wobbly positions will introduce an enormous variability in the real world laboratory implementations of this test; the confusing nonspecific description in the Corman-Drosten paper is not suitable as a Standard Operational Protocol making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus.
3. The test cannot discriminate between the whole virus and viral fragments. Therefore, the test cannot be used as a diagnostic for intact (infectious) viruses, making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus and make inferences about the presence of an infection.
4. A difference of 10° C with respect to the annealing temperature Tm for primer pair1 (RdRp_SARSr_F and RdRp_SARSr_R) also makes the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus.
5. A severe error is the omission of a Ct value at which a sample is considered positive and negative. This Ct value is also not found in follow-up submissions making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus.
6. The PCR products have not been validated at the molecular level. This fact makes the protocol useless as a specific diagnostic tool to identify the SARS-CoV-2 virus.
7. The PCR test contains neither a unique positive control to evaluate its specificity for SARS-CoV-2 nor a negative control to exclude the presence of other coronaviruses, making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus.
8. The test design in the Corman-Drosten paper is so vague and flawed that one can go in dozens of different directions; nothing is standardized and there is no SOP. This highly questions the scientific validity of the test and makes it unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus.[66]
They conclude that, “we have identified concerning errors and inherent fallacies which render the SARS-CoV-2 PCR test useless.”[67] The site is worth visiting, as they have links to many other articles exposing the flaws with the SARS-CoV-2 PCR test that make it unsuitable for detecting SARS-CoV-2.
If issues with the testing protocol are not enough, PCR tests also cannot distinguish between live virus/active infection and dead viral nucleotides. Therefore, even an accurate positive PCR test result may simply indicate the presence of viral remnants but not active nor transmissible infection. PCR tests also cannot measure viral load (actual amount of virus present in the host). These are further reasons the PCR is completely unreliable as a test for COVID-19, and this invalidates the results of clinical trials that depended upon the PCR for COVID-19 diagnosis.
Unfortunately, despite all of the shortcomings of the PCR test, this faulty protocol, officially recommended by the WHO[68], has driven the response to COVID-19. People without symptoms have been getting tested to try to avoid “asymptomatic transmission”. However, even according to Anthony Fauci, asymptomatic transmission has never been a driver in the outbreak of a respiratory virus[69]. A study of almost ten million people in Wuhan found no evidence of asymptomatic transmission.[70] Yet testing of asymptomatic people throughout the outbreak has resulted in an inflated view of reported “cases”. Even with symptomatic disease, is it possible that false positive COVID-19 PCR tests are resulting in other illnesses going undiagnosed or misdiagnosed?
For the 2020-2021 flu season, the CDC is unable to provide its annual in-season influenza burden estimate, a disease that in the U.S. has for decades caused hundreds of thousands of hospitalizations and tens of thousands of deaths annually[71]. Why? According to the CDC as of December 3, 2020, “The model used to generate influenza in-season preliminary burden estimates uses current season flu hospitalization data. Reported flu hospitalizations are too low at this time to generate an estimate.”[72] How is it that a virus so prevalent every year just disappears? Some argue that the measure taken to prevent the spread of COVID-19 (masks, social distancing) contributed to a major decline in flu cases, but this is unlikely. The WHO, in a 2019 study on non-pharmaceutical interventions to combat pandemic influenza states that, “There is also a lack of evidence for the effectiveness of improved respiratory etiquette and the use of face masks in community settings during influenza epidemics and pandemics”[73] (other pages in the report also state that there is little evidence to support the effectiveness of masks in slowing the spread of influenza). Likewise, the CDC in 2020 published a study in their journal, Emerging Infectious Diseases, that states:
In our systematic review, we identified 10 RCTs that reported estimates of the effectiveness of face masks in reducing laboratory-confirmed influenza virus infections in the community from literature published during 1946–July 27, 2018. In pooled analysis, we found no significant reduction in influenza transmission with the use of face masks (RR 0.78, 95% CI 0.51–1.20; I2 = 30%, p = 0.25) (Figure 2).
…
Disposable medical masks (also known as surgical masks) are loose-fitting devices that were designed to be worn by medical personnel to protect accidental contamination of patient wounds, and to protect the wearer against splashes or sprays of bodily fluids (36). There is limited evidence for their effectiveness in preventing influenza virus transmission either when worn by the infected person for source control or when worn by uninfected persons to reduce exposure. Our systematic review found no significant effect of face masks on transmission of laboratory-confirmed influenza.[74]
Since respiratory viruses generally spread from symptomatic infection, the probability that social distancing, especially of only 6 feet (aerosolized virus can travel much further than 6 feet and remain in the air for long periods of time)[75] has had any effect on virus transmission is minimal at best.
Therefore, it is unlikely that measures taken to slow the spread of COVID-19 (which have not been shown to have had any effect on the spread of COVID-19) has any effect on influenza cases. What happened to all the influenza? A virus with such a high annual burden doesn’t just disappear, so the answer is clear – influenza cases were diagnosed as COVID-19, likely due to false positive PCR tests, and patients were not tested for influenza. What this means is that the “case” numbers we’ve seen from the start have been greatly exaggerated. Even the numbers of those who have died from (or with – there is a big difference, and the latter is likely higher than the former) COVID-19 has also been greatly exaggerated. The fact is, the disease is likely not nearly as contagious as was originally projected, and that it is not nearly as dangerous, most likely posing the most hazard to those advanced in age and with complicating underlying health or immunity conditions.
Safety of the COVID-19 Vaccines
Are the shots safe? Though the CDC and all of the manufacturer websites claim the shots are “safe and effective”, on what premise do they base this claim? We know the CDC has already acknowledged and confirmed the shots can cause thrombosis with thrombocytopenia (TTC), blood clots with low platelet count, myocarditis, pericarditis, Guillane-Barre Syndrome, anaphylaxis, and other adverse reactions including death.[76] These are, as the document’s title expresses, “select” adverse events, meaning, it does not cover all adverse events. Granted, some adverse events are mild and likely no different than mild side-effects from other vaccines and medications. The CDC does, however, try to downplay the serious adverse events, more often than not calling them “rare.” What do the statistics really say? According to the Vaccine Adverse Events Reporting System (VAERS) database, there have been more reports of vaccine-related death from the COVID-19 shots than from all other vaccines since the system was instituted. A search of the VAERS system at https://wonder.cdc.gov/vaers.html for all deaths on all dates for all vaccines, as of Friday, July 30, 2021[77], produces a rather long table of data which includes the following summarized rows*:
* This summary table was created from data returned by a search of the VAERS database from the CDC Wonder site, keeping intact the erroneous calculations returned by the site.
** For some reason, the VAERS search engine on the CDC Wonder site is miscalculating a total of 10,672 events against which to calculate percentages. Actual percentages calculated against the correct total of 16,784 would, in order of the above summarized table, be: 3.25%, 16.93%, 16.49%, 0.17%, 63.16%, and 100% .
Based on corrected percentages, reports of death for the COVID-19 shots, available only for the last 7 or so months, account for just under 37% (36.84% to be precise) of all vaccine-related deaths reported to VAERS since 1990. In over 30 years of recording deaths related to vaccines, all other vaccines combined don’t account for even twice the number of deaths associated with the COVID-19 shots that have only been around for half a year. That should open some eyes. Compared to any other individual vaccine, the Pfizer and Moderna vaccines have each been reported as causing nearly four times more deaths than the next highest vaccine (Orimune Oral Polio Virus vaccine at 787).[78] Looking at reported adverse events other than death reveals a similar story. It should be known also that “’[u]nderreporting’ is one of the main limitations of passive surveillance systems, including VAERS.”[79] While severe adverse events are less likely to be underreported than minor side-effects, reporting is dependent upon the patient, patient’s family, or doctor to recognize or correlate the relationship between the patient receiving a vaccine with the adverse event. For all reported adverse events other than death, the Pfizer and Moderna vaccines come in at 204,003 and 196,019 respectively.[80] The next closest to these is the Varivax Varicella vaccine (varicella vaccines also have questionable effectiveness) at 75,682. There are 15 other vaccines with the number reported adverse effects ranging from 20,084 to 69,982. Of the remaining 209 vaccines listed, only 19 have had greater than 10,000 adverse events reported. That means adverse events for each of the other 190 vaccines are less than 5% of those for the two top COVID-19 shots. More attentive monitoring may contribute to some of the increased number of reports seen with the COVID-19 shots, especially if reactions occurred with participants of clinical trials, but that would not be enough to account for the vast difference in the number of reported events between the COVID-19 shots and other vaccines.
The FDA also has a “[w]orking list of at least 16 possible adverse events of special interest (AESI)”[81] that includes:
· Acute myocardial infarction
· Anaphylaxis
· Appendicitis
· Bell’s Palsy Narcolepsy
· Deep Vein Thrombosis (DVT)
· Disseminated intravascular coagulation (DIC)
· Encephalomyelitis
· Guillain-Barré syndrome
· Hemorrhagic Stroke
· Immune thrombocytopenia (ITP)
· Myocarditis/Pericarditis
· Non-hemorrhagic Stroke
· Pulmonary Embolism (PE)
· Thrombosis with Thrombocytopenia
· Transverse Myelitis
That is a pretty serious list. Further, a study out of the Penn Medicine Center for Evidence-based Practice published a meta-analysis of phase 1 and 2 clinical trials of several of the vaccines and found that, “[s]evere systemic adverse events were reported by 5 to 10 percent of trial subjects.”[82] 5 to 10 percent is quite a high number for severe adverse events, orders of magnitude higher than the chances of dying from severe COVID-19 infection.
Aside from monitoring for adverse events, how much safety testing has actually gone into the shots? According to Peter Doshi, certainly not enough:
Officials have consistently emphasised that despite shaving years off traditional timelines for producing vaccines, no compromises in the process were taken.20 However one type of study, tracking the distribution of a vaccine once injected in the body, was not conducted using any of the three vaccines currently authorised in the US.
Such biodistribution studies are a standard element of drug safety testing but “are usually not required for vaccines,” according to European Medicines Agency policy,21 which adds, “However, such studies might be applicable when new delivery systems are employed or when the vaccine contains novel adjuvants or excipients.”[83]
Where are studies on distribution and safety of the lipid nano-particles (LNPs) being used as the transport agent for the mRNA vaccines? What about the safety of the polyethylene glycol (PEG 2000 – a petroleum product) being used to stabilize the LNPs and aid in their absorption into cells? Many people don’t realize that a commonly prescribed OTC laxative is PEG of a particular molecular weight (PEG 3350), but complete safety studies have not been performed on it, as it is assumed to be safe because it is expected only minimally to be absorbed in the digestive tract. At least one manufacturer who produces “[b]iotechnology grade” PEG 2000, and on whose site the vaccines are mentioned, states this product is “NOT for human or animal use.”[84] It has been shown that LNPs are able to cross the blood-brain barrier via intramuscular injection.[85] Where are the studies showing the shots do not travel to the brain and cause potential issues? What adjuvants are being used in the vaccines, and what studies have been performed on the safety of these adjuvants?
Aside from all of the preceding safety concerns, there have been no long-term safety studies regarding these shots. Perhaps it will be found that someone who suffers no adverse event now may suffer issues later due to long-term elevated IgG and T cell titers. Perhaps someone does not have a severe adverse reaction within a week or two of the shot, but later suffers from accumulation of LNPs or uncleared spike protein in parts of the body other than the injection site. Perhaps an accumulation of one of the adjuvants or the LNPs causes a reaction as concentrations increase or is found to have a long-term toxic or carcinogenic effect.
There are too many potential risks and unknowns, and far too little study information for medical authorities to be making confident assertions regarding the safety of these shots.
Why the Strong Push to Get Everyone Vaccinated?
If there are so many risks, and the benefits are as yet unproven, why then is there such a strong push to get people to receive a medicine that hasn’t yet actually been approved by any country’s regulatory agencies? Why was information suppressed regarding palliative or ameliorative outpatient treatments, especially inexpensive ones that have been in use for a long time, like hydroxychloroquine and ivermectin, and are known to be safe? For that matter, why were there no official protocols for outpatient treatment? Why was the only promoted option for the ill to stay home until symptoms became severe and required hospitalization? When in history have doctors en masse ignored potential outpatient treatments while awaiting development of a novel vaccine? Why did the government and the news continuously assert that the only way to handle COVID-19 was developing a vaccine? Dr. Peter McCullough, internist, cardiologist, epidemiologist, and professor of medicine, who has testified before the Texas state legislature regarding the COVID-19 vaccines in an interview recorded on rumble.com asks many of these same questions: https://rumble.com/vhp7y5-full-interview-world-renowned-doctor-blows-lid-off-of-covid-vaccine.html . One need not don a tinfoil hat and claim Bill Gates wanting to depopulate the world as the reason for the push to find an answer. Just follow the money.
Pharmaceutical companies have been cashing in on these shots to the tune of billions of dollars. Even CNN would not hide this fact.[86] There is big money in vaccines, and very little liability. Though you hear otherwise on the news, vaccine development and manufacturing is the only industry in the U.S. for which the manufacturer cannot be sued if their product, even when used properly and as intended, harms someone.[87] Specifically, “In March 2020, the Secretary issued a PREP Act Declaration covering COVID-19 tests, drugs and vaccines providing liability protections to manufacturers, distributors, states, localities, licensed healthcare professionals, and others identified by the Secretary (qualified persons) who administer COVID-19 countermeasures.”[88] How many businesses are privileged enough to operate on all profit with no liability for their product? If the vaccines do cause harm, do you know who foots the bill for the liability? The American taxpayers pay any reparations for vaccine injuries. Though the COVID-19 shots are not covered under the National Vaccine Injury Compensation Program (funded by taxpayer dollars),[89] they are covered under the Countermeasures Injury Compensation Program[90] which is also funded by taxpayer dollars. So, we the people get to pay for any injuries caused by the vaccines while pharmaceutical companies hold on to the billions of dollars in vaccine revenues.
The current narrative is that a 95% vaccination rate needs to be reached in order to achieve “herd immunity.” Considering that, when Arthur Hedrich first applied the concept of herd immunity to observations regarding naturally acquired immunity to measles he stated that only 68% of the population (55% by some sources) needed to be infected and acquire immunity in order to protect the rest of the population, one must wonder from where the 95% figure arises. Rather than a portion of the population with acquired immunity protecting the rest of the community, a 95% vaccination rate is simply mass vaccination. To conflate this with the originally observed concept of herd immunity is completely illogical. There is some explanation for this inflated figure being forwarded as necessary to herd immunity, and that is the rate of vaccine failure. Serge Gregoire does a respectable job of explaining this in an article titled, Vaccine Failure: the Achilles’ heel of herd immunity.[91] Frustratingly enough, not only was the idea of herd immunity coopted to include vaccine-induced “immunity,” the WHO recently changed their definition of herd immunity to not even include naturally acquired immunity.[92]
In light of the evidence, it appears that fear continues to be stoked in order to increase the profits of the pharmaceutical companies by scaring people into participating, like lab rats, in what should be considered the largest human drug trial in history.
Conclusion
While vaccines may have their place in preventive medicine, the COVID-19 shots have been rushed into use far too quickly, and there are still too many questions about effectiveness and safety. There is no reason to believe the majority of the population needs to receive these experimental shots in order to stop a virus whose level of threat has been overestimated due to faulty testing, especially when safe and inexpensive outpatient treatments are available. I must agree with the conclusion at which the Doctors for COVID Ethics arrived:
…the benefits of vaccination are highly doubtful. In contrast, the harm the vaccines do is very well substantiated, with more than 15.000 vaccination-associated deaths now documented in the EU drug adverse events database (EudraVigilance), and over 7.000 more deaths within the UK and the US .
ALL PHYSICIANS MUST RECONSIDER THE ETHICAL ISSUES SURROUNDING COVID-19 VACCINATION.[93]
End Notes
[1] Oxford Dictionary of Biochemistry and Molecular Biology. “Vaccine”. Oxford Reference. Oxford University Press. Copyright 2021. https://www.oxfordreference.com/view/10.1093/oi/authority.20110803115026742
[2] Lexico, Powered by Oxford. “Vaccine”. Oxford English and Spanish Dictionary, Synonyms, and Spanish to English Translator. Lexico. Copyright 2021. https://www.lexico.com/en/definition/vaccine
[3] Sourced from National Center for Immunization and Respiratory Diseases. “Vaccines: The Basics”. Vaccines and Preventable Diseases. Centers for Disease Control and Prevention. March 14, 2012. https://www.cdc.gov/vaccines/vpd/vpd-vac-basics.html
The definition at this link has since been changed in order to allow the COVID shots to fit into the definition.
[4] Moderna. “What is the Moderna COVID-19 Vaccine”. moderna. July 2021. https://www.modernatx.com/covid19vaccine-eua/recipients/moderna-vaccine
[5] Pfizer Inc. “MRNA – Technology at the Forefront During Global Pandemic”. Pfizer. May 2021.
https://www.pfizer.com/news/hot-topics/mrna_technology_at_the_forefront_during_global_pandemic
[6] Janssen Therapeutics, Division of Janssen Products, LP. “The Janssen COVID‑19 Vaccine: How It’s Designed”. Janssen|Pharmaceutical Companies of Johnson & Johnson. July 2021. https://www.janssencovid19vaccine.com/hcp/how-its-designed.html
[7] AstraZeneca. “Discovery: Viral vectored technology”. AstraZeneca. Copyright 2021. https://covid19.astrazeneca.com/en/discovery.html
[8] National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. “Key Things to Know About COVID-19 Vaccines: Availability of Vaccines”. CDC. June 25, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/keythingstoknow.html
[9] AstraZeneca. “Package leaflet: Information for the user”. COVID-19 Vaccine AstraZeneca solution for injection. AstraZeneca. April 2021. https://www.covax.azcovid-19.com/content/dam/azcovid/pdf/covax/who-clean-pl-azd1222-en.pdf
[10] Janssen Therapeutics, Division of Janssen Products, LP. “About the Janssen COVID-19 Vaccine: What is the Janssen COVID-19 Vaccine”. Janssen|Pharmaceutical Companies of Johnson & Johnson. July 2021. https://www.janssencovid19vaccine.com/for-recipients/about-the-vaccine.html
[11] Janssen Therapeutics, Division of Janssen Products, LP. “The Janssen COVID‑19 Vaccine: How It’s Designed”. Janssen|Pharmaceutical Companies of Johnson & Johnson. July 2021. https://www.janssencovid19vaccine.com/hcp/how-its-designed.html
[12] Moderna. “What is the Moderna COVID-19 Vaccine: Why Is the Vaccine Available Now”. moderna. July 2021. https://www.modernatx.com/covid19vaccine-eua/recipients/moderna-vaccine
[13] Janssen Therapeutics, Division of Janssen Products, LP. “The Janssen COVID-19 Vaccine: How It’s Designed”. Janssen|Pharmaceutical Companies of Johnson & Johnson. July 2021. https://www.janssencovid19vaccine.com/for-recipients/about-the-vaccine.html
[14] AstraZeneca. “COVID-19 Vaccine AstraZeneca”. AstraZeneca. Copyright 2021. https://www.covax.azcovid-19.com/covax/xc/en/consumer.html
[15] Moderna. “Moderna COVID-19 Vaccine”. moderna. July 2021. https://www.modernatx.com/covid19vaccine-eua/
[16] Pfizer. “Coronavirus Disease (COVID-19) Resources”. Pfizer. Copyright 2020-2021. https://www.pfizer.com/science/coronavirus
[17] Pfizer Inc. “MRNA – Technology at the Forefront During Global Pandemic”. Pfizer. May 2021.
https://www.pfizer.com/news/hot-topics/mrna_technology_at_the_forefront_during_global_pandemic
[18] National Center for Immunization and Respiratory Diseases. “Vaccines & Immunizations: Development of New Vaccines”. Centers for Disease Control and Prevention. May 2014. https://www.cdc.gov/vaccines/basics/test-approve.html
[19] National Center for Immunization and Respiratory Diseases. “Vaccines & Immunizations: Vaccine Product Approval Process”. Centers for Disease Control and Prevention. May 2014. https://www.cdc.gov/vaccines/basics/test-approve.html
[20] Doshi, Peter. “Covid-19 vaccines: In the rush for regulatory approval, do we need more data?”. BMJ 2021; 372:n1244. May 18, 2021. https://doi.org/10.1136/bmj.n1244
[21] Ibid.
[22] Ibid.
[23] US Food and Drug Administration. 161st Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting. 2020. https://www.fda.gov/media/143982/download
[24] BioNTech SE. “Study to Describe the Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Individuals”. U.S. National Library of Medicine. April 30, 2020. https://clinicaltrials.gov/ct2/show/NCT04368728
[25] ModernaTX. “A Study to Evaluate Efficacy, Safety, and Immunogenicity of mRNA-1273 Vaccine in Adults Aged 18 Years and Older to Prevent COVID-19”. U.S. National Library of Medicine. July 14, 2020. https://clinicaltrials.gov/ct2/show/NCT04470427
[26] Janssen Vaccines & Prevention B.V. “A Study of Ad26.COV2.S for the Prevention of SARS-CoV-2-Mediated COVID-19 in Adult Participants (ENSEMBLE)”. U.S. National Library of Medicine. August 10, 2020. https://clinicaltrials.gov/ct2/show/NCT04505722
[27] AstraZeneca. “Phase III Double-blind, Placebo-controlled Study of AZD1222 for the Prevention of COVID-19 in Adults”. U.S. National Library of Medicine. August 18, 2020. https://clinicaltrials.gov/ct2/show/NCT04516746
[28] BoiNTech SE. “Study to Describe the Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Individuals”. U.S. National Library of Medicine/ClinicalTrials.gov. April 30, 2020. https://clinicaltrials.gov/ct2/show/NCT04368728
[29] Fernando P. Polack, M.D, et al. “Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine”. The New England Journal of Medicine. December 31, 2020. https://www.nejm.org/doi/full/10.1056/NEJMoa2034577#article_references
[30] Ibid.
[31] Ibid.
[32] Van Beusekom, M. “Phase 3 trials show AstraZeneca COVID vaccine has up to 90% efficacy”. Center for Infectious Disease Research and Policy. December 8, 2020. https://www.cidrap.umn.edu/news-perspective/2020/12/phase-3-trials-show-astrazeneca-covid-vaccine-has-90-efficacy
[33] Under the section titled “Vaccine Efficacy” in an article titled, “How to read results from COVID vaccine trials like a pro,” Adrian Esterman, Professor of Biostatistics and Epidemiology, University of South Australia writes:
One measure is based on the “attack rate”, which is the proportion of the people in the trial diagnosed with COVID-19. We measure the attack rate in the vaccine arm and the placebo arm separately, then divide one by the other to give the “attack rate ratio”. We then subtract the attack rate ratio from 1 to get one measure of vaccine efficacy.
For example, if 5% of the vaccine arm are diagnosed with COVID-19, while 40% of the placebo are diagnosed, then the attack rate ratio is (5%/40%) or 0.125 or 12.5%. That gives a vaccine efficacy of 87.5% (100% - 12.5%).
Esterman, Adrian. “How to read results from COVID vaccine trials like a pro”. The Conversation. November 18, 2020. https://theconversation.com/how-to-read-results-from-covid-vaccine-trials-like-a-pro-149916
[34] Mitchell, Sue. “Coronavirus doctor’s diary: Unvaccinated patients with many regrets”. BBC News. July 17, 2021. https://www.bbc.com/news/stories-57866661
[35] Vogel, A.B., Kanevsky, I., Che, Y. et al. “BNT162b vaccines protect rhesus macaques from SARS-CoV-2”. Nature 592, 283–289. February 1, 2021. https://doi.org/10.1038/s41586-021-03275-y
[36] Klimpel GR. ‘Immune Defenses: IgG Antibodies”. Medical Microbiology. 4th edition. 1996. https://www.ncbi.nlm.nih.gov/books/NBK8423/
[37] Ibid, under the section “Viral Activation of Immunity: Humoral Immunity”.
[38] Ibid, under the section “Antibody-Mediated Reactions: Production and the roles of antibody classes”.
[39] Ibid.
[40] Ibid.
[41] Doctors for COVID Ethics. “Studies published between May and July 2021 show pre-existing memory-type antibody responses to SARS-CoV-2 and COVID-19 vaccines”. Doctors for COVID Ethics. July 9, 2021. https://doctors4covidethics.org/letter-to-physicians-four-new-scientific-discoveries-crucial-to-the-safety-and-efficacy-of-covid-19-vaccines/
[42] United States Food and Drug Administration. “Antibody Testing Is Not Currently Recommended to Assess Immunity After COVID-19 Vaccination: FDA Safety Communication”. U.S. Food & Drug Administration. May 19, 2021. https://www.fda.gov/medical-devices/safety-communications/antibody-testing-not-currently-recommended-assess-immunity-after-covid-19-vaccination-fda-safety
[43] Nägele, M. P., Haubner, B., Tanner, F. C., Ruschitzka, F., & Flammer, A. J. (2020). “Endothelial dysfunction in COVID-19: Current findings and therapeutic implications”. Atherosclerosis, 314, 58–62. October 14, 2020. https://doi.org/10.1016/j.atherosclerosis.2020.10.014
[44] University of Nebraska Medical Center. “How long do mRNA and spike proteins last in the body?”. Nebraska Medicine. July 2, 2021. https://www.nebraskamed.com/COVID/where-mrna-vaccines-and-spike-proteins-go
[45] Lei, Y., Zhang, J., Schiavon, C. R., He, M., Chen, L., Shen, H., Zhang, Y., Yin, Q.,Cho, Y., Andrade, L., Shadel, G. S., Hepokoski, M., Lei, T., Wang, H., Zhang, J., Yuan, J. X.-J., Malhotra, A., Manor, U., Wang, S., Yuan, Z.-Y., and Shyy, J. Y.-J. “SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2”. Cirulation Research. April 30, 2021. https://www.ahajournals.org/doi/epub/10.1161/CIRCRESAHA.121.318902
[46] Klimpel GR. “Immune Defenses: Cell-Mediated Immunity: Cytotoxic T Lymphocytes”. Medical Microbiology. 4th edition. 1996. https://www.ncbi.nlm.nih.gov/books/NBK8423/
[47] Ibid.
[48] Vogel, A.B., Kanevsky, I., Che, Y. et al. “BNT162b vaccines protect rhesus macaques from SARS-CoV-2”. Nature 592, 283–289. February 1, 2021. https://doi.org/10.1038/s41586-021-03275-y
[49] Ibid.
[50] Nechipurenko, Y. D., Anashkina, A. A., & Matveeva, O. V. “Change of Antigenic Determinants of SARS-CoV-2 Virus S-Protein as a Possible Cause of Antibody-Dependent Enhancement of Virus Infection and Cytokine Storm”. Biophysics, 65(4), 703–709. October 19, 2020. https://doi.org/10.1134/S0006350920040119
[51] Lee, W.S., Wheatley, A.K., Kent, S.J. et al. “Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies”. Nature Microbiology 5, 1185–1191. September 9, 2020. https://doi.org/10.1038/s41564-020-00789-5
[52] Doctors for COVID Ethics. “Studies published between May and July 2021 show pre-existing memory-type antibody responses to SARS-CoV-2 and COVID-19 vaccines”. Doctors for COVID Ethics. July 9, 2021. https://doctors4covidethics.org/letter-to-physicians-four-new-scientific-discoveries-crucial-to-the-safety-and-efficacy-of-covid-19-vaccines/
[53] National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. “Selected Adverse Events Reported after COVID-19 Vaccination”. US Centers for Disease Control and Prevention. July 26, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html
[54] Vogel, A.B., Kanevsky, I., Che, Y. et al. “BNT162b vaccines protect rhesus macaques from SARS-CoV-2”. Nature 592, 283–289. February 1, 2021. https://doi.org/10.1038/s41586-021-03275-y
[55] Doshi, Peter. “Will covid-19 vaccines save lives? Current trials aren’t designed to tell us”. BMJ 2020;371:m4037. October 21, 2020. https://doi.org/10.1136/bmj.m4037
[56] Bernal, J.L., Andrews, N., Gower, C., Gallagher, E., Simmons, R., Thelwall, S., Stowe, J., Tessier, E., Groves, N., Dabrera, G., Myers, R., Campbell, C. N. J., et al. "Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant". The New England Journal of Medicine. July 21, 2021. https://www.nejm.org/doi/full/10.1056/NEJMoa2108891
[57] Wharton, M., Chorba, T., Vogt, R., Morse, D., Buehler, J. “Case Definitions for Public Health Surveillance”. CDC. May 02, 2001. https://www.cdc.gov/mmwr/preview/mmwrhtml/00025629.htm
[58] US Centers for Disease Control and Prevention. “Outbreak Case Definitions”. CDC. (no date). https://www.cdc.gov/urdo/downloads/casedefinitions.pdf
[59] Ibid.
[60] National Human Genome Research Institute. “Polymerase Chain Reaction (PCR) Fact Sheet”. National Human Genome Research Institute. August 17, 2020. https://www.genome.gov/about-genomics/fact-sheets/Polymerase-Chain-Reaction-Fact-Sheet
[61] US Centers for Disease Control and Prevention. “CDC 2019-Novel Coronavirus (2019-nCoV)
Real-Time RT-PCR Diagnostic Panel”. US Food & Drug Administration. December 1, 2020. https://www.fda.gov/media/134922/download
[62] US Food & Drug Administration. “FAQs on Testing for SARS-CoV-2”. US Food & Drug Administration. March 25, 2021. https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/faqs-testing-sars-cov-2
[63] National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. “CDC’s Diagnostic Test for COVID-19 Only and Supplies”. US Centers for Disease Control and Prevention. July 13, 2021. https://www.cdc.gov/coronavirus/2019-ncov/lab/virus-requests.html
[64] Division of Laboratory Systems. “07/21/2021: Lab Alert: Changes to CDC RT-PCR for SARS-CoV-2 Testing”. US Centers for Disease Control and Prevention. July 21, 2021. https://www.cdc.gov/csels/dls/locs/2021/07-21-2021-lab-alert-Changes_CDC_RT-PCR_SARS-CoV-2_Testing_1.html
[65] Corman Victor M, Landt Olfert, Kaiser Marco, Molenkamp Richard, Meijer Adam, Chu Daniel KW, Bleicker Tobias, Brünink Sebastian, Schneider Julia, Schmidt Marie Luisa, Mulders Daphne GJC, Haagmans Bart L, van der Veer Bas, van den Brink Sharon, Wijsman Lisa, Goderski Gabriel, Romette Jean-Louis, Ellis Joanna, Zambon Maria, Peiris Malik, Goossens Herman, Reusken Chantal, Koopmans Marion PG, Drosten Christian. “Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR”. Euro Surveillance 2020;25(3):pii=2000045. January 22, 2020. https://doi.org/10.2807/1560-7917.ES.2020.25.3.2000045
[66] Borger, P., Malhotram, B. R., Yeadon, M., Craig, C., McKernan, K., Steger, K. , McSheehy, P., Angelova, L., Franchi, F., Binder, F., Ullrich, H., Ohashi, M., Scoglio, S., Doesburg-van Kleffens, M., Gilbert, D., Klement, R., Schruefer, R., Pieksma, B. W., Bonte, J., Dalle Carbonare, B. H., Corbett, K. P., Kämmerer, U. “Review report Corman-Drosten et al. Eurosurveillance 2020”. Corman-Drosten Review Report. February 2020. https://cormandrostenreview.com/report/
[67] Ibid.
[68] Drosten, C., et. al. “Diagnostic detection of 2019-nCoV by real-time RT-PCR”. World Health Organization. January 17, 2020. https://www.who.int/docs/default-source/coronaviruse/protocol-v2-1.pdf?sfvrsn=a9ef618c_2
[69] CNN Special Reports. “CNN Special Report, COVID WAR: The Pandemic Doctors Speak Out. Aired 9-10p ET”. CNN. March 28, 2021. https://transcripts.cnn.com/show/csr/date/2021-03-28/segment/02
[70] Cao, S., Gan, Y., Wang, C. et al. Post-lockdown SARS-CoV-2 nucleic acid screening in nearly ten million residents of Wuhan, China. Nat Commun 11, 5917. November 20, 2020. https://doi.org/10.1038/s41467-020-19802-w
[71] Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases. “Past Seasons Estimated Influenza Disease Burden”. US Centers for Disease Control and Prevention. October 1, 2020. https://www.cdc.gov/flu/about/burden/past-seasons.html
[72] Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases. “2019-2020 U.S. Flu Season: Preliminary In-Season Burden Estimates”. US Centers for Disease Control and Prevention. December 3, 2020. https://www.cdc.gov/flu/about/burden/preliminary-in-season-estimates.htm
[73] World Health Organization. “Non-pharmaceutical public health measures for mitigating the risk and impact of epidemic and pandemic influenza”, page 4. World Health Organization. 2019. https://apps.who.int/iris/bitstream/handle/10665/329438/9789241516839-eng.pdf
[74] Xiao, J., Shiu, E., Gao, H., Wong, J. Y., Fong, M. W., Ryu, S....Cowling, B. J. “Nonpharmaceutical Measures for Pandemic Influenza in Nonhealthcare Settings—Personal Protective and Environmental Measures”. Emerging Infectious Diseases, 26(5), 967-975. May 2020. https://doi.org/10.3201/eid2605.190994
[75] “Aerosols, on the other hand, are tiny by comparison, nearly 10,000 times smaller than a human hair. They’re spread at far greater distances—20 to 30 feet—and can linger in the air for minutes to hours, infecting others.” – Barber, Carolyn. “Protecting Against COVID’s Aerosol Threat”. Scientific American. October 1, 2020. https://www.scientificamerican.com/article/protecting-against-covids-aerosol-threat/
[76] National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. “Selected Adverse Events Reported after COVID-19 Vaccination”. US Centers for Disease Control and Prevention. July 26, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html
[77] https://wonder.cdc.gov/vaers.html . Report was generated with the following parameters entered into the Request Form:
1. Organize table layout: Group Results By: Vaccine
2. Unchanged
3. Unchanged
4. Select location, age, gender: The United States/Territories/Unknown (this is selected by default)
5. Select other event characteristics: Event Category: Death
6. Unchanged
7. Unchanged
8. Unchanged
9. Unchanged
10. Unchanged
11. Unchanged
12. Unchanged
At the time the search was submitted, there was an error in the percentage calculations returned by the site. Data is updated weekly in the VAERS database. Searches run after July 30, 2021 may produce different results.
[78] Ibid.
[79] US Department of Health and Human Services. “Guide to Interpreting VAERS Data”. Vaccine Adverse Event Reporting System. https://vaers.hhs.gov/data/dataguide.html
[80] https://wonder.cdc.gov/vaers.html . Report was generated with the following parameters entered into the Request Form:
1. Organize table layout: Group Results By: Vaccine
2. Unchanged
3. Unchanged
4. Select location, age, gender: The United States/Territories/Unknown (this is selected by default)
5. Select other event characteristics: Event Category: All events other than Death selected
6. Unchanged
7. Unchanged
8. Unchanged
9. Unchanged
10. Unchanged
11. Unchanged
12. Unchanged
At the time the search was submitted, there was an error in the percentage calculations returned by the site. Data is updated weekly in the VAERS database. Searches run after July 30, 2021 may produce different results.
[81] Anderson, Steve. “FDA Updates of COVID-19 Vaccine Safety Activities.” U.S. Food & Drug Administration. June 10, 2021, slide 8. https://www.fda.gov/media/150051/download
[82] Mull, N. K., Mitchell, M. D., Brennan, P. J. “Adverse Effects of Messenger RNA Vaccines”. Penn Medicine (University of Pennsylvania). December 2020, p1. http://www.uphs.upenn.edu/cep/COVID/mRNA%20vaccine%20review%20final.pdf
[83] Doshi, Peter. “Covid-19 vaccines: In the rush for regulatory approval, do we need more data?” BMJ 2021;373:n1244. May 18, 2021. https://www.bmj.com/content/373/bmj.n1244
[84] Molecular Depot. “Polyethylene glycol 2000 (PEG 2000)”. Molecular Depot. https://moleculardepot.com/product/polyethylene-glycol-2000-peg-2000/
[85] Fernandes, F., Dias-Teixeira, M., Delerue-Matos, C., & Grosso, C. “Critical Review of Lipid-Based Nanoparticles as Carriers of Neuroprotective Drugs and Extracts”. Nanomaterials (Basel, Switzerland), 11(3), 563. February 24, 2021. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7996241/
[86] Ziady, Hanna. “Covid vaccine profits mint 9 new pharma billionaires”. CNN Business. May 21, 2021. https://www.cnn.com/2021/05/21/business/covid-vaccine-billionaires/index.html
[87] U.S. House of Representatives. “42 USC 300aa-22: Standards of responsibility”. United States House of Representatives. https://uscode.house.gov/view.xhtml?path=&req=granuleid%3AUSC-prelim-title42-section300aa-22&f=&fq=&num=0&hl=false&edition=prelim
[88] U.S. Department of Health & Human Services. “PREP Act Immunity from Liability for COVID-19 Vaccinators”. Public Health Emergency. April 13, 2021. https://www.phe.gov/emergency/events/COVID19/COVIDvaccinators/Pages/PREP-Act-Immunity-from-Liability-for-COVID-19-Vaccinators.aspx
[89] Health Resources & Services Administration. “National Vaccine Injury Compensation Program”. HRSA. July 2021. https://www.hrsa.gov/vaccine-compensation/index.html
[90] Health Resources & Services Administration. “Countermeasures Injury Compensation Program”. HRSA. November 2020. https://www.hrsa.gov/cicp/
[91] Serge Gregoire. “Vaccine failure: the Achilles’ heel of herd immunity”. Dr. Serge, The Nutrition Scientist. January 9, 2020. https://www.drsergegregoire.com/vaccines/vaccine-failure-the-achilles-heel-of-herd-immunity/
[92] Tara. “Why Did the WHO Alter Its Definition of ‘Herd Immunity’?”. Medium. December 23, 2020. https://allswritewiththeworld.medium.com/why-did-the-who-alter-its-definition-of-herd-immunity-d701abeb5a77
[93] Doctors for COVID Ethics. “Studies published between May and July 2021 show pre-existing memory-type antibody responses to SARS-CoV-2 and COVID-19 vaccines”. Doctors for COVID Ethics. July 9, 2021. https://doctors4covidethics.org/letter-to-physicians-four-new-scientific-discoveries-crucial-to-the-safety-and-efficacy-of-covid-19-vaccines/


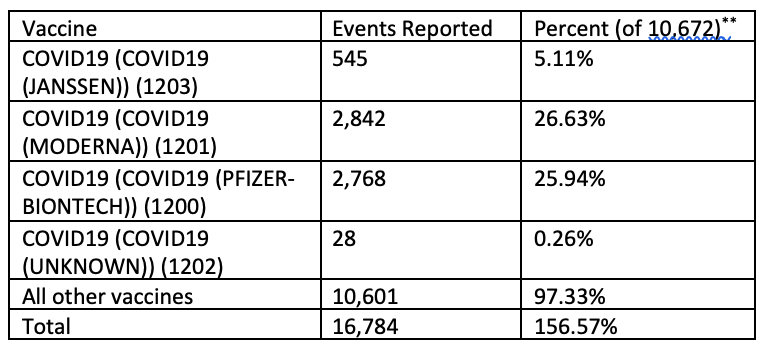
Comprehensive!